
Newsletter January 2019


|
Newsletter January 2019 |

|
Pharmacovigilance and social networksPharmacovigilance and social Media Monitoring, Why?Today, only 5% of side effects concerning drugs and other health products are reported to health authorities. This is why one of the major challenges of pharmacovigilance is to involve patients more strongly in the pharmacovigilance process because the signals are very weak and therefore underestimated. A solution? Search for pharmacovigilance cases on social networks by Social Monitoring. How to integrate pharmacovigilance in the age of social networks?Based on a report published by the ANSM (French National Agency of Health and Drug)
The percentage of cases reported by patients has not only remained constant, but above all very low. Is it due to a lack of communication of professionals? Or means offered to patients? Knowing that today online forms and applications have been made available to report adverse reactions. A study has shown that patients tend to increasingly turn to the web and social networks to share their health experiences. But is it really relevant to use social media for pharmacovigilance? Can social media monitoring have a beneficial and substantial effect on pharmacovigilance? We have noticed that there is a real tendency for patients to share adverse reactions with other patients or health professionals on blogs, forums, patient communities, social networks and this in a very natural and transparent way. There are pots, photos, comments, tweets on different themes such as diabetes, cancer, allergies, depression, pain, different STDs, vaccination etc. The volume and quality of this information would be substantial and richer because it comes directly from patients and in near real time. In order to be able to raise patient awareness and place them in the center of pharmacovigilance, it is necessary to go where they are, to be able to listen to them, detect potential risks and inform them. On the other hand, the measures related to the web and social networks are multiplying, there are still no decrees or laws, but directives from the European Union, the United States and the United Kingdom. Finally, the fundamentals of social networks are similar to those of Pharmacovigilance (listen, spread and engage) and the potential benefits are promising...
How will this data be collected, analysed and used?The studies conducted have demonstrated that there are more adverse reactions described on forums than on other web sources because forum users have the opportunity to write more text. On Twitter, on the other hand, there is more "noise" about the adverse effects observed, but often for advertising purposes. All sources do not have the same level of quality, often forums in English are more easy to explore than forums in other languages. There is also the problem of detecting abbreviations and translating the patient language into medical language which is not an obvious thing, so the challenge is to successfully detect cases of unreported adverse reactions. Also, succeed in differentiating between real and fake cases. Some laboratories have begun to conduct studies on patient behaviour using information that circulates between patients on social networks Specific dictionaries have been designed to deal with the nature and language used, and automated semantic analysis methods to recognize signs/symptoms and emotions experienced by patients. Ex: "I don't think we should..." = pessimistic confidence on the part of the patient. Patients express themselves more easily and naturally online than in front of healthcare professionals. Thus the pathologies are analysed in greater depth: signs/symptoms - Biology - Examinations. Today these signals are taken into consideration and the authorities take care to verify the data and process them. Especially since they consider that the turn of phrase and the language of the patients can be interesting. Pharmacovigilance cannot yet be done because patients remain anonymous, but pharmacoepidemiology is quite possible thanks to these signals. The different issuesFirst there is the question on the representativeness of the sample on the Internet Could it become a substitute? Interesting to see a new way of doing things Absence of barriers on the Internet (age, education etc.) so it gives a certain legitimacy There is an evolution between those who conduct the study on social networks and those who use it Source of recruitment of patients with rare diseases: the rarer the pathology is, the greater the need to talk about it on the Internet Is it really worth using this channel? Regulatory authorities: the acquisition of data using the non-traditional method is ethical and NICTs are more appropriate. |
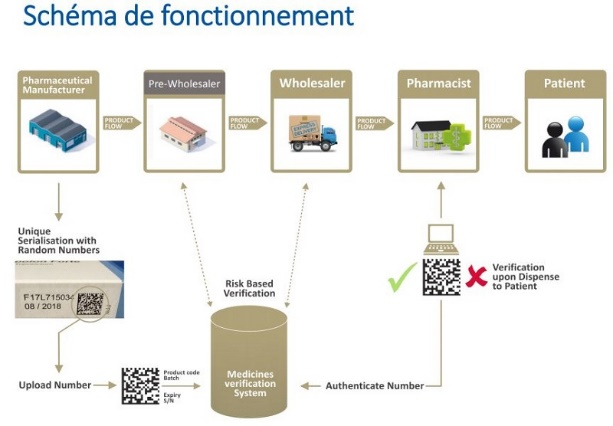
What will change in 2019 as part of the European directive to fight against counterfeit medicines (LEEM)?Serialization: A new traceability device will be placed on the boxes of medicines circulating in Europe, to allow identification to the box with a unique number written in code form, so each box will be followed from its manufacture to its marketing! It will be operational in Europe from February 2019. 
Cyber Health: mHealth**!** Mobile Health One of the major innovations in clinical research is e-health; new medical devices are emerging such as wearable devices: Fitbits, Apple watch, smartphone applications are accelerating data collection while increasing patient participation and engagement. These tools should increase efficiency by extending lead times and reducing costs. This prevents patients from not attending their monitoring visits or dropping out in the middle of a clinical trial. According to Pharmaceuticals Research and Manufacturer, 94% of pharmaceutical companies, OCR, service providers plan to use Mobile Health (mHealth). The mHealth should be transformed into a generalized useBut these devices must meet FDA regulatory standards. Unlike an EDC tool, they are not designed to validate protocol changes and probably not compliant with CFR part 11. What should industry do to convince the FDA?It should demonstrate the effectiveness of these devices by implementing version control, by demonstrating that data collected through a smartphone application is aligned with data collected in an EDC or other measures. And also be able to ensure that the Patient is effectively the person using the wearable device. On the other hand, integration with a cloud solution can be a second solution, offering the possibility of real-time access to all stakeholders to vital information from participants and potentially involving more patients in trials. Many benefits are observed when using mHealth in combination with a cloud infrastructure: new opportunities for pharmaceutical researchers to collect data, increase the number of recruitments, and make trial participation much easier for patients. This will allow researchers to bring treatments that improve the quality of life to market more quickly! 
Transformations in clinical researchThe adoption of new technology in clinical research has made it possible to improve speed and efficiency. 3 elements have disrupted research from phase 1 to post-market surveillance: artificial intelligence, online clinical tools and mHealth. These have enabled researchers to overcome challenges related to patient recruitment and retention, data entry and analysis, etc. The FDA has raised the issue that industry is adopting new time-saving tools and new test models to further innovate and streamline testing. These developments advance the future of the clinical research industry, particularly with respect to research for oncology drugs and biologics in Phase 1 clinical trials. |
If you are interested in hearing about best practices from the pharmaceutical industry and learn more about eClinical software, please register at Medrio Explore
The main themes will be the Acceleration of Clinical Trials, with the different challenges and opportunities facing clinical researchers today. 
|
Animal Health - Drug Safety
The 2019 ACDM Annual Conference
The 2019 ACDM Annual Conference (ACDM19) will be held at the Radisson Blu Amsterdam Airport from the 11th to the 12th March 2019 There will ne more sessions, more speakers, more chance for delegates to network and visit the Exhibition and also enjoy the ‘Dinner Event’. Click here for more details and registration. Society for Clinical Data Management
|

|
We regularly improve our newsletter based on our opinion but also based on the feedback we receive, in order to ensure you will always enjoy the content. However we are conscious that you are busy. So if you have any comment or suggestion, please feel free to use the form below to contact us
|